Ozone occurs in the upper and lower atmosphere. The term ozone depletion refers to the thinning of the ozone in the upper atmosphere or stratosphere in particular. This phenomenon is caused by anthropogenic emissions of chemicals that destroy the ozone layer. Halogen source gases that contain chlorine and bromine, which break down in the upper atmosphere, destroy stratospheric ozone. Chlorofluorocarbons, historically used in aerosol propellants, are one example of an ozone depleting chemical. The chemical reactions that occur with reactive chlorine and bromine molecules have depleted ozone levels most significantly at the poles, specifically over Antarctica. An image provided by NASA in 2006 revealed a shocking hole in the ozone layer 11.4 million square miles wide over the Antarctic. 1 (see image below)
Effects of Ozone Depletion
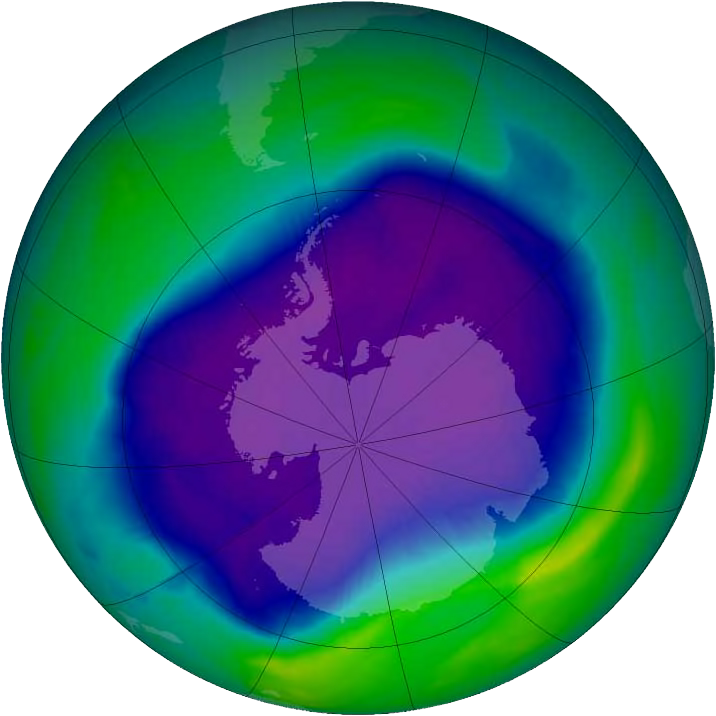
Ozone in the upper atmosphere has a positive effect. It plays a significant role in the stratosphere by protecting Earth’s surface from dangerous solar radiation in the form of ultraviolet or UV-B rays which can adversely affect humans, plants and animals. Therefore, ozone depletion contributes to increased ultraviolet radiation which negatively impacts all forms of life. As a result, humans are more susceptible to skin cancer like carcinoma, melanoma and cataracts. UV-B rays can harm fish and amphibian eggs, alter the DNA or proteins of plants and reduce crop yields. Marine food webs can also be disrupted by UV-B radiation. The ecological consequences of ultraviolet radiation are complex. In addition, chlorofluorocarbons, one source of ozone depletion, also contribute to climate change or what is known as the Greenhouse Effect. 2
History
The U.S. Environmental Protection Agency banned the “nonessential use of chlorofluorocarbons in aerosol sprays” in 1978 after research by chemists Mario Molina and Sherwood Rowland linked chlorofluorocarbons to the destruction of ozone. 3 The Antarctic ozone hole revealed by research raised concern and pressure from various environmental groups, leading to the institution of the Montreal Protocol on Substances that Deplete the Ozone Layer. This international treaty went into effect in 1989 to cut chlorofluorocarbon emissions to 50 percent of 1986 levels by 1998. The Copenhagen Amendment of 1992 modified the treaty by adding controls on hydro-chlorofluorocarbons and speeding up deadlines to reduce ozone-depleting chemicals. Tighter regulations on these chemicals led to more research on developing substitutes for chlorofluorocarbons and hydrofluorocarbons. As a result, the total concentration of ozone depleting chemicals has dropped since the 1990’s. Stratospheric ozone levels outside the polar regions have stabilized. 4
Ethics
Stratospheric ozone depletion raises an ethical issue in regards to the diffusion of harm. No single individual is responsible for ozone depletion. Instead, the actions of many corporations and individuals producing and using these chemicals have created the problem. Therefore, it is difficult to assign blame to any one person or any single group of people. We must decide “Do corporations and nations have the same moral responsibilities as individuals? When harm is caused by the joint actions of many parties, how should responsibility be allocated?” 5
Another ethical issue arises in the fact that the harm caused by these chemicals was previously unknown, unintentional and indirect. The damage was indirect because the chemicals themselves were not damaging the atmosphere. Instead, it was the effects of these substances on the ozone that proved harmful. Distributing blame for this problem is far from simple because the producers of these chemicals were also unaware of the potential harmful effects of their chemicals. How does one decide if people should be held responsible for harm they did not know they were causing? The nature of the issue raises complex ethical questions that cannot easily be answered. 6
